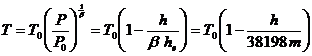Within the Troposphere
This theoretical model makes several assumptions about our atmosphere:
1. Air behaves as an ideal gas (PV=nRT). At these low pressures (0 to 1 atm), this is an easy assumption. The properties of N2 & O2 gases do not differ measurably from that of diatomic ideal gases. This assumes that there are no chemical reactions or photo-dissociations.
2. The acceleration due to gravity, g, is constant. At 11 km of altitude, g differs from sea level by only 0.3%.
3. Air is free to move vertically but without any additional heat being added (adiabatic). This implies that the air is completely transparent to radiant energy and that conduction is negligible. This is probably fairly accurate except near the ground and in the presence of clouds. The latent heat of condensation, and the radiant heat absorbed or reflected by clouds can make an appreciable difference here.
Assume that air is an ideal gas.

Replace V:
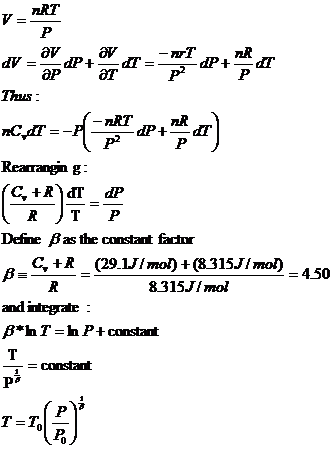
From the Constant Temperature model, before actually using the assumption of constant T the following equation was derived. It assumed that the air was an ideal gas with an average molar mass of 28.8 g/mol and that pressure was due to supporting the weight of air.
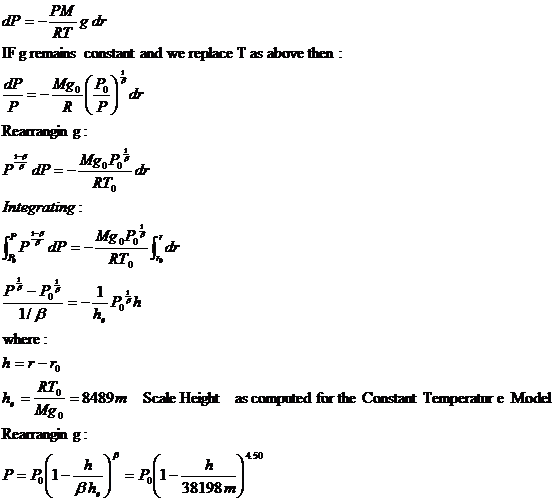
Using this expression for P and substituting it into the original equation for T