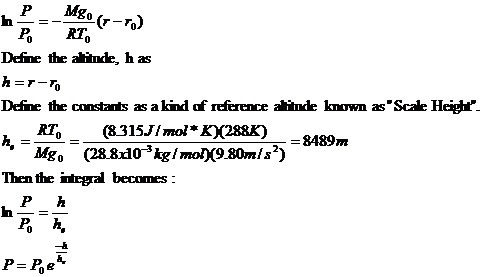Within the Troposphere
This theoretical model makes several assumptions about our atmosphere:
1. Air behaves as an ideal gas (PV=nRT) with a molecular mass of 28.8 g/mol. At these low pressures (0 to 1 atm), this is an easy assumption. The properties of N2 & O2 gases do not differ measurably from that of ideal gases. The weighted average of the molecular masses of these two gases is 28.8 g/mol.
2. The acceleration due to gravity, g, is constant. At 11 km of altitude, g differs from sea level by only 0.3%.
3. Pressure is due solely to supporting the weight of air. This assumes that the atmosphere is static with no forces accelerating masses of air. During clear weather this is usually not to unreasonable.
4. Because of constant mixing, the temperature of the air remains a constant throughout. This turns out to be the least reasonable assumption. Not only do the surface temperatures vary frequently through a range of up to 100°C but even the average temperature of the air varies with the altitude (0 to 11km) over a range of 70°C.
Assume that air is an ideal gas.

Pressure is due to the weight of the air.
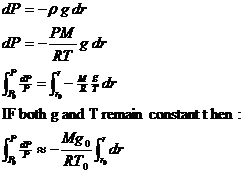
Integrating: